Wastewater Treatment through Chemical and Physical Processes
Wastewater treatment uses chemical and physical processes to solve specific problems. Screens, strainers and membrane technology, for instance, are employed to separate solids. Processes such as precipitation and nanofiltration remove heavy metals from wastewaters.
Process Combination for Wastewater Treatment

Depending on the composition of the wastewater, the chemical and physical treatments often take place in individual steps. DAS Environmental Expert effectively combines these process steps with the right wastewater technology as an efficient and cost-effective solution for the treatment of the client’s wastewater.
After selecting the ideal combination of procedures, our experts in project management will accompany you during the planning and construction of your plant.
DAS offers Chemical and Physical Water and Wastewater Treatment Processes for every Area of Application
Wastewaters containing water insoluble substances or colloids are effectively treated through processes such as sedimentation, filtration and centrifugal separation. Flotation, where substance particles stick to fine air bubbles due to adhesive forces is another process that, depending on the wastewater’s composition, DAS Environmental Expert often uses as part of the physical treatment stage. Reliable, mechanical preliminary cleaning is particularly important for the treatment of sanitary wastewaters in order to prevent damage in the subsequent treatment stages.
Chemical wastewater treatment forces contaminants that are dissolved in wastewater to separate more easily through the targeted addition of specific substances. During precipitation, a previously dissolved substance is turned into a dissoluble substance that can be filtered from the liquid. Other methods of pollutant removal are ion exchange, flocculation, UV and ozone treatments.
Physical Wastewater Treatment Processes
Coarse-and-Fine Materials Separation Utilizing Screens and Strainer
Screens and strainer remove solid contaminants from wastewater. These mechanical processes separate solid pollutants such as diapers, hair, and wet wipes from the wastewater stream. Before the treatment of industrial wastewaters, strainer separate textile fibers, paper labels, plastic residuals, and production residues such as potato peels and other scraps and wastes.
Depending on the area of application, coarse or fine screens are used. They clean the wastewater by means of parallel rods. Strainer feature grits, screens, perforations and meshes of varying sizes. Coarse strainer (> 20 mm) to micro (<0.05 mm) separate solid substances as large as human waste to as small as sand and tiny sludge particles from the wastewater stream.
Extremely important is the mechanical preliminary cleaning in the treatment of sanitary wastewaters. Fibers suspended in the wastewater pose a particular challenge, especially the extremely tear-resistant textile fibers of wet wipes and non-woven materials. They tend to build up, potentially creating blockages and enormous damage to pumps and mixers.
DAS Environmental Expert works closely with its clients when choosing the right drum screens and self-cleaning screens to prevent damage to their treatment technology. This eliminates unnecessary maintenance from the outset and saves costs.

Mechanical Separation of Solid Substances through Filtration
Filtration separates solid substances from fluids. To this end, the mixture passes through a filter made of paper; whereas, technical applications typically utilize filters made from textiles or metal. Sand filters, cloth filters and drum screens are also frequently used as filtration systems.
Filtration systems remove organic and inorganic suspended solids, sands and dusts from wastewater. Wastewater technology employs this mechanical separation process to drain sludge in filter presses, among other processes. Filtration, typically in multistage processes, is also used for the purification of surface water to provide domestic and potable water.
Membrane filtration is another mechanical separation process in which a membrane functions as the filter medium. This method is typically used to separate very fine particles.
Wastewater Treatment through Membrane Technology
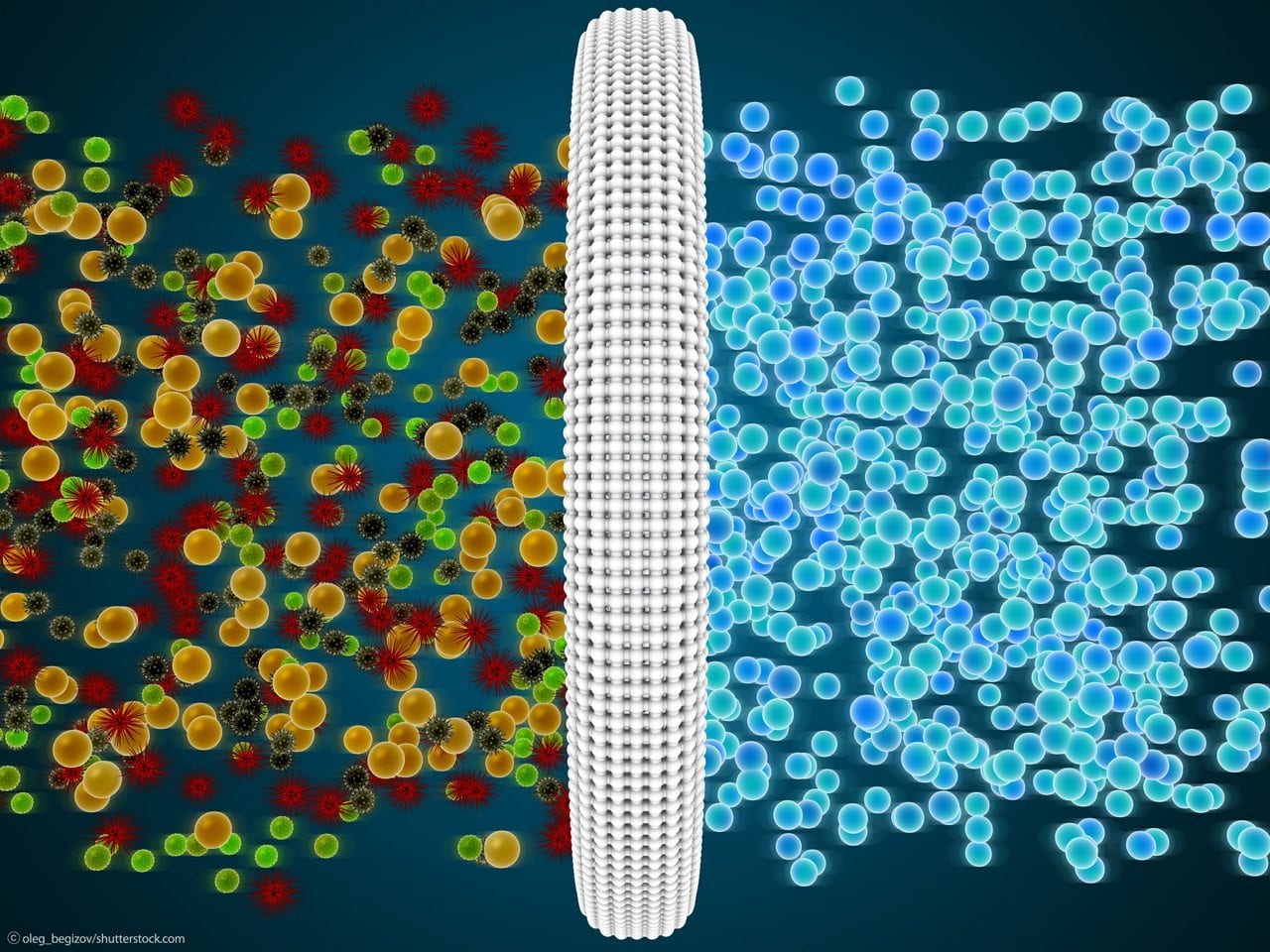
Membrane filtration separates and concentrates dissolved and un-dissolved substances from wastewater. This separation is performed under pressure. Due to its specific pore size, the membrane retains particles and molecules of a certain size. Different methods of membrane filtration are used for water purification, wastewater treatment, process water recycling, and the collection of recyclables in the recovery of valuable substances.
Microfiltration is employed to separate particles, bacteria and yeasts. It is also used for cold sterilization and for the separation of oil-water emulsions.
Ultrafiltration is an important method for the treatment of wastewater and potable water. It serves to separate particles, microorganisms, proteins and turbidities from the water. Ultrafiltration is used in the Membrane Activation Reactor (MBR).
For instance, ultrafiltration is used to clean water in swimming pools. Since the build up of clogging deposits on the membrane can be prevented, more and more pre-existing wastewater treatment systems are being complemented with ultrafiltration as a final step. When retrofitting older wastewater treatment plants, the ultrafiltration step can be positioned directly inside or as a separate stage after the activation tank in order to replace subsequent treatment steps or to increase the treatment capacity of the biological wastewater treatment.
Nanofiltration retains viruses, heavy metal ions, large molecules and very fine particles. The method is used for water softening and the treatment of potable water.
Reverse Osmosis is an important process to concentrate landfill wastewaters, treat potable water in rural areas that are not connected to a pipeline network, desalinate seawater and decalcify boiler water in power plants. This method concentrates substances that are dissolved in fluids by applying pressure through a semi-permeable membrane that reverses the process of osmosis. When the applied pressure is higher than the respective osmotic pressure, the molecules of the solvent diffuse to the side of the membrane where the dissolved substance is already less concentrated. Reverse osmosis is also used to produce ultrapure water.
Wastewater Treatment through Flotation
Flotation removes dispersed or suspended substances from fluids by means of very fine gas bubbles that transport the substances to the surface, and subsequently, bubbles and substances are removed with a clearing device. In wastewater treatment, the flotation processes are used to separate oils, fats and finely suspended solids and particles.
The smaller the micro-bubbles, the better the accumulation of particles or droplets function. To this end, wastewater technology often uses Dissolved Air Flotation (DAF), a method proven to be economically efficient. In addition, auxiliary agents such as collectors, frothers, controllers and pushers support flotation processes.

Solids Separation through Sedimentation
Sedimentation uses gravity to separate solid particles in sedimentation tanks. A sedimentation tank is a flat, nearly current-free tank specifically designed for sedimentation processes. The solid particles settle on the bottom of the tank.
Wastewater treatment uses sedimentation processes in various ways. In the preliminary cleaning tank, un-dissolved substances settle and form primary sludge that is subsequently concentrated in the digestion tower where it is transformed anaerobically. The transformation process produces digested sludge and fermentation gas, which, in its cleaned form like biogas, is converted into electricity to cover energy demands. Aerobically produced sludge is also added to the digestion tower after it has been separated from the wastewater through sedimentation in the clarifier tank. In addition, sand traps and sludge collectors separate particles that are heavier than water.
Wastewater Treatment through Chemical Processes
Neutralization
Wastewater technology uses neutralization to adjust the pH value. Acids or alkali are added, as required, after processes like precipitation and flocculation, and for the neutralization of industrial wastewaters.
Oxidation/Reduction
Redox reactions are frequently utilized in chemical wastewater treatment and in the treatment of potable water. Oxidation processes with ozone and hydrogen peroxide efficiently remove chlorinated hydrocarbons and pesticides from potable water.
In wastewater treatment, oxidation processes are used to remove difficult biodegradable compounds. Particularly efficient is photochemical purification, which forms hydroxyl radicals from hydrogen peroxide or ozone through UV-light exposure. These Advanced Oxidation Processes (AOP) are used to degrade drug substances like antibiotics, cytostatic drugs, hormones and other anthropogenic trace substances.
In addition, ozone aids in the oxidization of iron and manganese in well water. Reduction processes are required to transform heavy metal ions, for instance, into easily dissoluble sulfides.
Adsorption and Chemisorption
Adsorption is the accumulation of substances on the surface of a solid body, which is a physical process where molecules stick to boundary surfaces through the van der Waal force. If chemical bonding binds substances to the surface of a solid body, the process is called chemisorption. In contrast to adsorption, chemisorption is non-reversible.
Wastewater treatment uses activated carbons to bind soluble water contents that could not be sufficiently removed with lower-priced methods such as biological wastewater treatment, precipitation and flocculation. Colorants from textile dying plants, for instance, often can only be completely removed through adsorption on activated carbon. Anthropogenic trace elements such as pharmaceutical residues and polar organic substances like adsorbable, organically-bound halogens (AOX) also bind to activated carbon.
Doped activated carbon can also be employed to remove arsenic and heavy metals. Granulated iron hydroxide is another ideal agent to remove toxic metalloid arsenic from potable water, contaminated ground water and industrial wastewaters. In this process, the iron hydroxide reacts with the arsenate ions to form iron arsenate. This method is efficient as well as cost-effective.
Precipitation
Precipitation is a chemical process that separates a previously soluble substance from a fluid. A common method is to create a precipitation reaction by adding suitable agents. Through precipitation, heavy metals, for instance, transform to not easily soluble metal hydroxides. Other situations may require precipitation to carbonates or sulfides.
Anions can often be precipitated as calcium, iron, and aluminum salts. The separation of fluoride ions, for instance, is achieved through precipitation with milk of lime. During wastewater treatment in the treatment plant, adding salts like iron(II) sulfate, iron chloride or aluminum chloride lowers the phosphate concentration. The phosphate precipitation can either be integrated as simultaneous precipitation into the biological treatment stage or added as a subsequent separate process step.
Flocculation
Flocculation prepares very fine particles that are present either suspended or in the form of colloidal solutions, for removal from water. If the surface charge of this very fine particulate matter is the same, the particles cannot, due to mutual electrical repulsion, accumulate to larger agglomerates.
In this case, suitable chemicals, flocculants and flocculation aids help achieve the agglomeration of such particulate matter, creating macro flakes that sediment. Flocculation is used to improve settling properties as well as to drain sewage sludge. Employing iron and aluminum salts for flocculation allows the flocculating of phosphate at the same time.
Ion Exchanger
Ion exchangers are materials that can replace the ions of one solution with other ions. The cation exchanger, for instance, replaces calcium ions with sodium ions. Once the ion exchange is exhausted and the calcium ions are completely saturated, the ion exchanger needs regeneration.
This success of this process is based on the principle of displacement: the higher the ions’ charge, the stronger the ion-binding to the ion exchanger. If both types of ions are charged the same, the one with the larger radius will be the one with the stronger ion-binding force. During the ion exchange process, the stronger-binding ion will displace the lesser-binding ion.
Ion exchangers are suitable for the removal of heavy metals and anions and are therefore often used as ‘policing filters’ after precipitation and flocculation. In addition, they assist with water softening, changing the water’s salt content, and water desalination; particularly important for the semiconductor industry that uses them to produce extremely clean, demineralized water known as ultra pure water.